近日,2023年第31屆國際血栓與止血學(xué)會(huì)(ISTH)年會(huì)在加拿大魁北克蒙特利爾舉行。作為首位在ISTH會(huì)議上就中國自主研發(fā)產(chǎn)品做大會(huì)演講的中國臨床PI,中國醫(yī)學(xué)科學(xué)院血液病醫(yī)院楊仁池教授以口頭報(bào)告形式公布一項(xiàng)有關(guān)晟斯生物的超長效重組凝血因子產(chǎn)品注射用培重組人凝血因子VIII-Fc融合蛋白的臨床研究結(jié)果,為全球一百多萬患者帶來更多更好的選擇,讓患者不僅能解除病痛,更能回歸正常的生活。

滿足臨床需求,提升患者用藥依從性
血友病是一組凝血因子缺乏導(dǎo)致凝血功能障礙的遺傳性出血性疾病,也是嚴(yán)重危害健康的出生缺陷疾病。患者臨床常表現(xiàn)為自發(fā)性出血或輕度外傷后出血不止,極易導(dǎo)致患者殘疾,嚴(yán)重者可危及生命,血友病患者也因此被稱為“玻璃人”。全球血友病患者超過一百萬人,其中中國血友病患者約有14萬人,這些患者都需要終身注射凝血因子。
第一代血友病治療藥物為血源性凝血因子制劑,主要來源為血漿提取,存在血源感染、供給不足等問題。上世紀(jì)九十年代問世的第二代重組凝血因子制劑可有效降低血源性病毒傳播的風(fēng)險(xiǎn),提升產(chǎn)品的安全性。隨著第三代長效凝血因子的出現(xiàn),將進(jìn)一步提升患者用藥依從性。
注射用培重組人凝血因子VIII-Fc融合蛋白(代號(hào):FRSW117)同時(shí)采用PEG修飾和Fc融合蛋白兩種長效技術(shù),成功實(shí)現(xiàn)“一周一次”預(yù)防治療,是世界第二款、國產(chǎn)第一款在研的超長效重組八因子產(chǎn)品,讓血友病A患者回歸正常生活。產(chǎn)品具有自主知識(shí)產(chǎn)權(quán),核心專利已經(jīng)在多個(gè)國家和地區(qū)授權(quán)或進(jìn)入實(shí)質(zhì)審查階段。
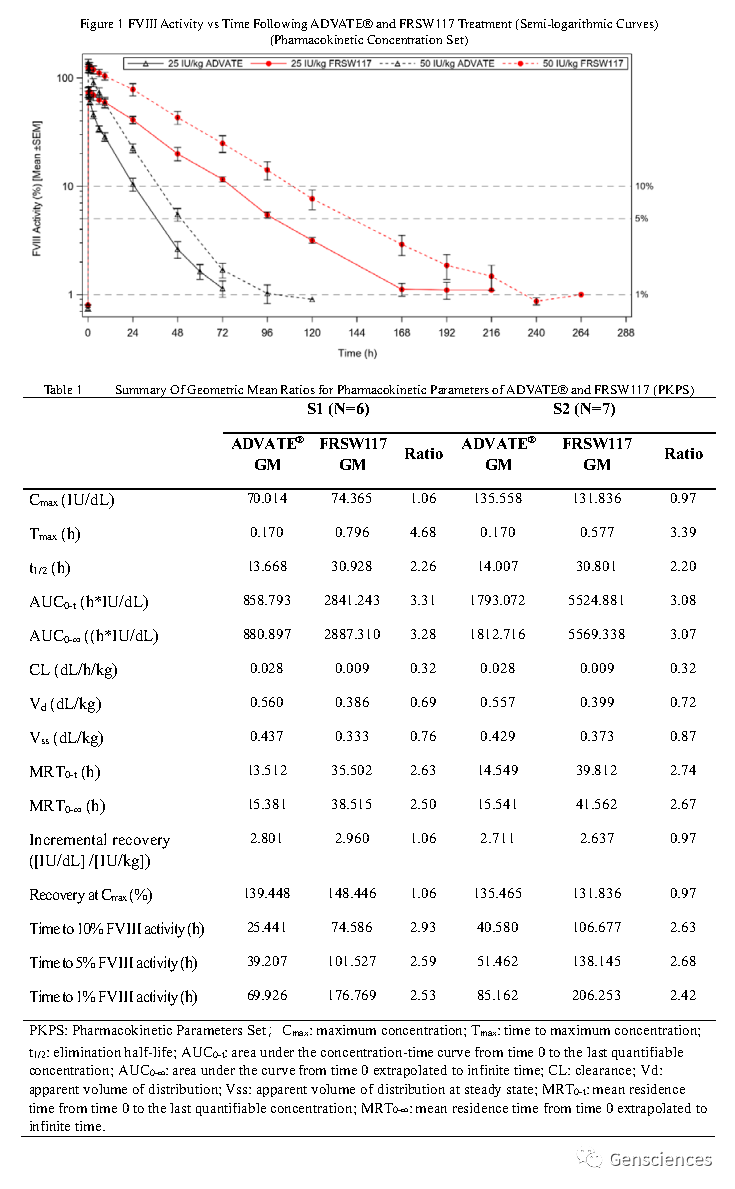
FRSW117可以實(shí)現(xiàn)一周一次的治療效果,極大改善了患者的生活狀況;在所有受試者中,未檢測到八因子抑制物的存在。臨床Ⅰ期研究數(shù)據(jù)顯示,不同劑量的兩組受試者(25 IU/kg和50 IU/kg)體內(nèi)維持在1%八因子活性以上的時(shí)間與現(xiàn)有的商業(yè)化產(chǎn)品(ADVATE?)相比,分別延長了2.59倍和2.49倍。同時(shí),兩組受試者體內(nèi)八因子的半衰期分別為ADVATE?產(chǎn)品的2.129倍和2.232倍。
Abstract原文如下:
A Phase 1 Study to Evaluate the PK, Safety and Tolerability of FRSW117 With Extended Half-Life in Patients With Severe Hemophilia A
NCT number: NCT04864743
Abstract ID: 1443652
OC 24 - Hemophilia Clinical – Factor Levels and Pharmacokinetics
Sunday, June 25, 2023,14:45 – 16:00, Eastern Time
Background: Factor VIII (FVIII) replacement therapy of severe hemophilia A (HA) necessitates multiple doses of FVIII products weekly to maintain a > 1% FVIII trough activity due to their short half-life. FRSW117 is a PEGylated FVIII Fc fusion protein engineered to extend its half-life so as to maintain a sufficient FVIII level and improve treatment outcomes.
Aims: To evaluate the pharmacokinetics, safety/tolerability, and immunogenicity of FRSW117 in patients with severe HA.
Methods: In this first-in-human, open label, single dose, self-controlled study, 13 previously treated males aged 12 (inclusive) to 65 years with severe HA (FVIII < 1%) were enrolled to receive ADVATE? followed by FRSW117, both at 25 IU/kg, 4 days apart (N=6, Group S1); or both drugs at 50 IU/kg, 6 days apart (N=7, Group S2), followed by a 4-week follow-up after FRSW117 injection. All patients provided written informed consents. The protocol was approved by each site’s IEC/IRB.
Results: The maximum concentration (Cmax) and area under the curve (AUC0-t) of FⅧ activity increased in a dose-dependent manner (Figure 1). The geometric mean elimination half-life of FRSW117 exceeded twice that of ADVATE? (30.928 hours vs. 13.668 hours in S1; 30.801 hours vs. 14.007 hours in S2) (Table 1). The mean FVIII level was > 5% for 101.527 hours in S1 and 138.145 hours in S2. Both groups maintained an FVIII activity of > 1% for longer than 168 hours (7 days), 176.769 hours for S1 and 206.253 hours for S2. No FⅧ inhibitors were detected and no hypersensitivity or anaphylaxis were reported. No serious adverse events or ≥ Grade 3 adverse events were reported. No spontaneous bleeding requiring intervention was reported within 9 days after FRSW117 treatment.
Conclusions: FRSW117’s pharmacokinetic characteristics supported a potential weekly treatment interval. It also displayed favorable safety/tolerability and immunogenicity profiles.